31 May, 2020
We wrote in early April to explain the types and functions of therapeutic and vaccines and tests, their pros and cons, attempting to tackle Covid-19. We continue to track global developments on these in real time for your reference here. Six weeks later, we are writing an update on what we have learnt more broadly scientifically in the intervening period and how our understanding is progressing.
This is the second in a three-part blog series:
I. Disease pathogenesis and risk factors
II. Therapeutic and vaccine progress
III. Viral mutation rate emerging strains and serology testing
1. Moderna, which was the first to enter the clinic with an mRNA based vaccine, has reported promising early data from its Phase I trial, but in results from a very small number of individuals so far. The vaccine was well tolerated and caused patients to develop an antibody response to the virus at comparable or higher levels to that seen in the plasma from recovered patients. The antibodies from 8 immunised individuals could neutralise the live virus in laboratory assays. The company hopes to start a Phase III trial in July.
2. CanSino Biologics, who entered the clinic in March with its recombinant adenovirus vaccine candidate, has published the results of a Phase I trial. The vaccine stimulated neutralising antibodies and a T cell response in patients however it is too early to know whether this confers protection. The company is currently undertaking Phase II trials.
3. The first anti-viral therapeutic, Remdesivir, has received FDA emergency approval. An early Phase II study using “triple combination” therapy of lopinavir-ritonavir (viral protease inhibitor), ribavirin (nucleoside analogue) and interferon beta-1b (a cytokine to stimulate the immune response) has shown promising early data that warrants further investigation at Phase III. Despite early endorsement there has been no definitive data on the efficacy of chloroquine and hydroxychloroquine.
4. The first neutralising antibody therapies plan to enter the clinic in June and large-scale convalescent plasma trials are underway in the UK and the US.
5. Innovative therapeutic approaches classes such as mesenchymal stem cell therapy to treat acute respiratory distress and recombinant ACEC2 proteins are being trialled.
The following section describes updates in the therapeutic and vaccine space since our last post in early April. For a summary and background of therapeutic strategies against Covid-19 please see our previous post here.
On 29th April the first emergency use approval was granted by the Food and Drug Administration (“FDA”) for Remdesivir – an antiviral drug developed by Gilead Sciences to treat Ebola. Early reports of trial data were confounding, with a study in China on 158 patients not showing statistical significant differences between the control and treatment groups [1]. Analysts pointed out the sample size was small due to challenges in recruiting patients as the epidemic waned in China, and the drug was delivered up to 12 days after symptom onset which is late in the disease development. An independent Phase II trial led by the National Institute of Allergy and Infectious Diseases (“NIAID”) concluded Remdesivir resulted in a 31% faster recovery time compared the placebo (11 days vs 15 days) [2], which prompted FDA engagement. Figure 1 shows the results published in the New England Journal of Medicine and shows an incremental but significant increase in survival of patients treated with Remdesivir compared to placebo[3].
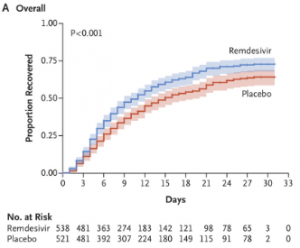
Figure 1: Comparison of Covid-19 patients treated with Placebo vs. Remdesivir. Source: Beigel et al., NEJM May 2020.
Encouragingly, a Phase II trial of “triple combination” therapy of lopinavir-ritonavir (viral protease inhibitor), ribavirin (nucleoside analogue) and interferon beta-1b (a cytokine to stimulate the immune response) on 86 treated patients in Hong Kong showed the median time to a negative test was reduced from 12 days to 7 days compared to the placebo group and warrants evaluation at Phase III (Figure 2)[4].
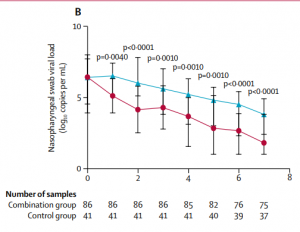
Figure 2: SARS-CoV-2 viral load in the placebo group (blue line) vs patients treated with lopinavir-ritonavir, ribavirin and interferon beta-1b. Source: Hung et al., Lancet 2020.
Chloroquine and Hydroxychloroquine received significant early endorsement, buoyed by initial positive reports and ready availability. Unfortunately, a recently published New England Journal of Medicine paper that investigated 1446 patients in New York indicated no significant association between hydroxychloroquine use and clinical outcome[5]. A study published in the Lancet that evaluated 14,888 treated patients compared to 81,144 controls could not confirm a benefit and found treatment was associated with increased risk of in hospital mortality and de novo ventricular arrhythmia[6]. Cardiac side effects following treatment with hydroxychloroquine and chloroquine have been reported in other studies[7]. On Monday, the 25th May the WHO confirmed they would be temporarily pausing trials involving hydroxychloroquine[8].
Many biotech companies and pharmaceutical companies have continued to develop antibody products to neutralise the virus, by targeting the viral Spike protein. Companies are favouring ‘cocktail-based’ approaches to avoid viral evasion through mutagenesis, and some are engineering the antibodies to reduce the risk of Antibody Dependent Enhancement (“ADE”), a phenomenon we wrote about in a previous blog. Regeneron anticipates its antibody cocktail will enter the clinic in June, AstraZeneca are selecting a candidate and plan to enter the clinic between July and September[9], Eli Lilly has formed partnership with AbCellera and Junshi Biosciences and Sorrento has formed a partnership with Mount Sinai in New York, to name a few of the ongoing efforts.
Convalescent plasma received emergency use FDA approval in late March and Phase III trials are due to begin in May. In late April, the UK Blood and Transplant service launched a widespread programme to collect convalescent plasma as part of a UK based trial called “REMAP-CAP,” which once scaled up will be able to treat 5,000 patients per week.
Over the last month more companies are pursuing cell therapies or innovative other therapeutic approaches. Mesoblast reported that its allogenic mesenchymal stem cell product resulted in 83% survival of severe patients in a small trial in New York[10] and is now preparing for a larger scale trial. Healios and VCanBio has also announced cell therapy based trials for ARDS patients[11],[12]. Apeiron Biologics is undertaking Phase II trials for its drug APN01 which is recombinant ACE2 protein, designed to act as a decoy to ACE2 receptors[13].
There are multiple ways to develop a vaccine, with the main approaches being:
Vaccine trials have been pushing ahead with Phase I trials underway or complete for at least 7 candidates as of the 18th May 2020[14]:
After demonstrating safety at Phase I, the vaccines must demonstrate efficacy or evidence of providing protection against SARS-CoV-2. Trials are set up so that one group of trial patients receives the vaccines and the other group receives a placebo. The outcome is then measured as the number of patients that are infected with SARS-CoV-2 over the coming months. There is a risk as the current wave subsides and levels of the virus reduce in the community that trials may need to run for longer, with larger patient groups to show significant efficacy. Companies also need to be flexible and consider rapidly setting up trials in different countries as viral waves take off. Professor Sarah Gilbert, who is leading the team behind the Oxford University Jenner Institute / Vaccitech vaccine said they plan to have enough data for regulatory approval by September. GSK who are partnering with Sanofi has said it believes Q4 2021 is realistic.
We are tracking therapeutic and vaccine updates, along with other positive developments here.
The third part of this three-part blog series will be coming shortly. Watch this space!
[1] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext
[2] https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
[3] https://www.nejm.org/doi/full/10.1056/NEJMoa2007764?query=recirc_curatedRelated_article
[4] https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(20)31042-4.pdf
[5] https://www.nejm.org/doi/full/10.1056/NEJMoa2012410
[6] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/fulltext
[7] https://www.sciencemag.org/news/2020/04/antimalarials-widely-used-against-covid-19-heighten-risk-cardiac-arrest-how-can-doctors
[8] https://www.npr.org/sections/coronavirus-live-updates/2020/05/25/861913688/who-halts-hydroxychloroquine-trial-over-safety-concerns?t=1590750077682
[9] https://www.ft.com/content/d31b9ce3-578f-41c3-853f-a0e7827ec79e
[10] https://www.forbes.com/sites/alexknapp/2020/05/02/large-scale-clinical-trials-of-mesoblasts-stem-cell-treatment-for-covid-19-coronavirus-set-to-begin-soon/#42447ec74086
[11] https://www.prnewswire.com/news-releases/covid-19-ards-patients-added-to-healios-one-bridge-study-301039906.html
[12] https://clinicaltrials.gov/ct2/show/NCT04288102
[13] https://www.clinicaltrialsarena.com/news/apeiron-biologics-phaseii-covid-19/
[14] https://blogs.sciencemag.org/pipeline/archives/2020/04/23/a-close-look-at-the-frontrunning-coronavirus-vaccines-as-of-april-23
[15] https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-interim-phase-1-data-its-mrna-vaccine
[16] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31208-3/fulltext
[17] https://science.sciencemag.org/content/early/2020/05/06/science.abc1932, https://medicalxpress.com/news/2020-04-chinese-biotech-firm-coronavirus-vaccine.html
[18] https://www.nytimes.com/2020/04/27/world/europe/coronavirus-vaccine-update-oxford.html
[19] https://www.biorxiv.org/content/10.1101/2020.05.13.093195v1.full.pdf
[20] https://www.fiercebiotech.com/biotech/astrazeneca-s-covid-19-vaccine-enters-phase-2-3-clinical-trial?mkt_tok=eyJpIjoiTVRrd1lUVm1NelkyWVRkaCIsInQiOiJabTlOQnM3RlgxVENcL1JRXC9tbUdHMHJVZDhPcGpqWDBqWTQrTGttU3RtMkpkaDZZeXZ0NzVzXC9PKzhLczZFaUx0Y2lqblZBM1wvZlZtOUhBeEpZQkZUdThJU2JYK0piM3dadmdnS3FcL2RORkN1R1JIVllcL1hUbW1LZk8xXC9naER5RFZUWnIyTlNQS1NnQUZXT2xrUXROc2hnPT0ifQ%3D%3D&mrkid=114702534
[21] https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-oxford-university-announce-landmark-agreement-for-covid-19-vaccine.html
[22] https://www.marketwatch.com/story/novartis-to-make-experimental-coronavirus-vaccine-2020-05-28
DISCLAIMER
This document and the information contained herein is for information purposes only. The document is provided on a non-reliance basis contains forward looking statements and opinions of the manager, which may be subject to change or revision without notice. Some information contained herein was based on, obtained or derived from data published or prepared by other parties (“Third-Party Information”).
None of Ahren or its affiliates, directors, officers, employees, partners, shareholders or agents (each, an “Ahren Party”) assumes any responsibility for the accuracy of the document or any Third-Party Information. No Ahren Party makes any representation or warranty, express or implied, as to the accuracy or completeness of this document or any Third-Party Information and no Ahren Party shall have any liability to the recipient or any other person relating to or resulting from the use of or reliance on any such information contained herein or any errors therein or omissions therefrom. No warranty, express or implied, or assurance is given by the manager or any of its directors, officers, members or employees.
Nothing contained in this document may be relied upon as a guarantee, promise, assurance or a representation as to the future. Except as otherwise indicated, the information provided in this Investor Letter is based on matters as they exist as of the date listed on the cover and not as of any future date, and will not be updated or otherwise revised to reflect information that subsequently becomes available or circumstances existing or changes occurring after the date hereof. The views expressed in this Investor Letter are subject to change based on market and other conditions. In considering the performance information contained herein, prospective investors should bear in mind that past, forecasted or targeted performance is not necessarily indicative of future results, and there can be no assurance that comparable results will be achieved.
Neither Ahren nor any of its affiliates makes any representation or warranty, express or implied, as to the accuracy or completeness of the information contained herein, and nothing contained herein should be relied upon as a promise or representation as to past or future performance of Ahren or the fund or any other entity.
The recipient acknowledges that, to the maximum extent permitted by law, each of Ahren and its related parties or affiliates disclaims all liability to the recipient or to any other person for any expense, cost, loss or damage of any kind including direct, indirect or consequential loss or damage (however caused, including by negligence) incurred by any person arising from or relating to any information included or omitted from this Investor Letter, whether by reason of such information being inaccurate or incomplete or for any other reason. This Investor Letter does not constitute and should not be considered as any form of financial opinion or recommendation. The recipient should conduct its own inquiries as to the adequacy, accuracy, completeness and reliability of any information, whether such information is contained in this Investor Letter or not, relating to Ahren or the fund.
No action should be taken by any recipient which, in the light of the information herein, would render such action illegal or violate any applicable laws or regulations. Neither the manager nor any of its directors, officers, members or employees accepts any liability whatsoever for its accuracy or any action taken by a recipient which is illegal or in violation of any applicable law or regulation as a result of receiving this document and its contents.
The Site cannot and does not contain medical or health related advice. The medical or health information is provided for general informational and educational purposes only and is not a substitute for professional advice. Accordingly, before taking any actions based upon such information, we encourage you to consult with the appropriate professionals. We do not provide any kind of medical or health advice.
The Site does not offer, and should not be construed in any way as offering legal, regulatory or tax advice. The information is provided for general informational purposes only and is not a substitute for professional legal or tax advice.
THE USE OR RELIANCE OF ANY INFORMATION CONTAINED ON THIS SITE IS SOLELY AT YOUR OWN RISK.
 22 December, 2020
Professor Zoubin Ghahramani: Ahren Podcast Series – A Glimpse into the Future with Ahren’s Science Partners
22 December, 2020
Professor Zoubin Ghahramani: Ahren Podcast Series – A Glimpse into the Future with Ahren’s Science Partners
 18 December, 2020
Ahren Innovation Capital’s Annual Symposium – Science Partner Panel Highlights 2019
18 December, 2020
Ahren Innovation Capital’s Annual Symposium – Science Partner Panel Highlights 2019